In my recent article on personalized medicine in oncology, I touched on the need for more and better clinical trials to make precision medicine a reality for cancer patients. There are currently several types of clinical trial designs for this purpose, all of which come with their own pros and cons. When diving deeper into this topic, it became clear that it requires and deserves more than one post, owing to the diversity and complexity of the different trial designs and their importance for improving the outcomes of cancer therapy. Thus, in this two-part series, I will go over some of these innovative, “adaptive” trial designs in more detail and discuss their potential in creating truly personalized medicine.
First Off, What is “Personalized Medicine”?
 As discussed here, personalized medicine (or “precision medicine”) refers to the use of genetic information to determine the best management strategy for each patient. To this end, there are numerous assays and tools. These include tumor DNA sequencing, standardized biomarker assays, radiomics, drug sensitivity assays, and companion diagnostics, just to name a few. Precision medicine can help determine the best antineoplastic agent for each patient in terms of efficacy and toxicity. Further, it can also determine if a person is at elevated risk for a certain cancer and would benefit from preventive measures such as lifestyle modifications or prophylactic surgery. The overall hope is that precision medicine will result in cancer patients achieving both better treatment outcomes and fewer side effects.
As discussed here, personalized medicine (or “precision medicine”) refers to the use of genetic information to determine the best management strategy for each patient. To this end, there are numerous assays and tools. These include tumor DNA sequencing, standardized biomarker assays, radiomics, drug sensitivity assays, and companion diagnostics, just to name a few. Precision medicine can help determine the best antineoplastic agent for each patient in terms of efficacy and toxicity. Further, it can also determine if a person is at elevated risk for a certain cancer and would benefit from preventive measures such as lifestyle modifications or prophylactic surgery. The overall hope is that precision medicine will result in cancer patients achieving both better treatment outcomes and fewer side effects.
All that said, the use of personalized medicine in oncology faces many challenges before it will become routine clinical practice. These include the need to screen a large number of patients to identify and enroll the population of interest in traditional clinical trials, since most molecular biomarkers have a low prevalence in cancer. Adaptive trials were developed to circumvent some of these issues, although these face obstacles of their own.
What are the Current Designs for Precision Medicine Clinical Trials?
There are currently numerous different biomarker-driven and adaptive designs being explored in oncology. For the sake of brevity, this article will focus on adaptive trials as a broad concept, while I will explore specific adaptive trial designs such as umbrella, basket, and N-of-1 trials in the second part.
Adaptive Trials
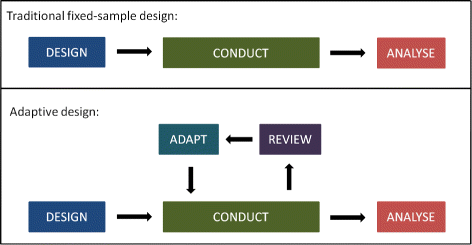
The US FDA defines an adaptive trial design as “a clinical trial design that allows for prospectively planned modifications to one or more aspects of the design based on accumulating data from subjects in the trial.”
Adaptive designs are applicable across all phases of clinical research. In theory, adopting an adaptive design can result in clinical trials becoming more flexible, efficient, informative, and ethical. This is because this design allows optimization of resource use and might require fewer participants. All adaptations should be specified at the design stage of the trial to allow calculation of operating characteristics such as the rate of type I error.
For example, adaptations can be made to the sample size, patient population, treatment arm selection, endpoint selection, and/or patient allocation ratio. There are also more complex adaptive designs such as Bayesian adaptive designs and adaptations based on multiple design features (e.g. multi-arm, multi-stage trials), biomarkers, time-to-event settings, simultations, and surrogate or intermediate endpoints. A more detailed look into each of these can be found here.
A good overview of adaptive trials from Amgen.
“Population Enrichment” Adaptive Trials
So-called “population enrichment” adaptive trials are one highly relevant clinical trial design for promoting personalized medicine in oncology. Oftentimes, the effect of a certain treatment will be greater in a targeted subset of the trial population. For example, patients with a particular clinicodemographic factor or genetic marker related to the drug’s mechanism of action may be more responsive to treatment. In these cases, a design that allows changes in the patient population and enrollment based on pre-specified criteria and the interim analysis results may be beneficial. Data collected both before and after the interim analysis can be combined to assess the treatment effect in the patient population of interest.
This type of trial design provides greater power at the same sample size than a fixed sample design in the overall population and furthermore allows evaluation of the treatment in the non-targeted subpopulation.
Advantages of Adaptive Trials
The theoretical advantages of adaptive trials are manifold, including (but not limited to) the following:
- They allow modification of the trial based on information that was not available at the time of trial initiation
- Greater statistical power
- They allow early termination of recruitment for, and fewer patients being randomized to, less promising treatment arms or doses. As a result, a larger proportion of patients may receive a more effective treatment.
- Enrolment of fewer trial participants, without negatively affecting the statistical power. In turn, this reduces or prevents wasting of resources (e.g. money, time).
- Identification of the patient subpopulation most likely to benefit from a treatment
- More precise estimation of treatment effects and dose-response or dose-toxicity relationships in specific populations or subpopulations, without the need for huge sample sizes
- Adaptive trials may be “more acceptable” to key stakeholders such as study sponsors and patients
- A definitive conclusion may be reached sooner, reducing the length of the trial and allowing earlier approval and access to new treatments
So Why are Adaptive Trials Not More Common?
On paper, adaptive trials sound far superior than their non-adaptive, traditional counterparts. Nevertheless, the uptake of adaptive clinical trial designs has been relatively slow, owing to several important hurdles. These vary depending on the specific design used and the adaptations made.
For example, some limitations to widespread use of adaptive clinical trial designs in oncology include:
- Lack of experience with these designs
- Non-harmonized terminology
- The work required to evaluate candidate designs
- Concerns about funding and how study sponsors, payers, and regulators view adaptive designs
- The need for buy-in to an adaptive design from regulators, including Institutional Review Boards
- Lack of understanding of the applicability, potential, and practical implications of adaptive designs
- The need for complex statistical methods to avoid making erroneous conclusions and minimize the risk of introducing bias
- Lack of clarity regarding the best ways to interpret and report the results
- The inherent complexity of adaptive trial study protocols, resulting in longer intervals between planning and starting the trial
- Challenges related to running the trial itself, such as drug supply requirements
- The added logistical challenges and need for additional steps in terms of ensuring appropriate trial conduct and trial integrity. This includes appropriately blinding and limiting access to the comparative interim results. Knowledge of the accumulating data can affect the course and conduct of a trial and the behaviors of the trial sponsor, investigators, and participants.
What Can We do to Accelerate the Uptake of Adaptive Trials?
The short answer is: lots of things! Of course, addressing the many limitations identified above has to be number 1. I could go into each of these in detail, but you would end up with a 10,000-word essay. Instead, I propose that most of these barriers can be overcome through a combination of increasing awareness, close communication and collaboration with all key stakeholders (especially statisticians!), and education. As with many other things, knowledge-sharing and discussions of what did and did not work, lessons learned, and practical experiences will help ensure competency and confidence in designing future adaptive trials. Whether the trial results are positive or negative, publication is also key so that the broader medical community can learn from past experiences.
Adaptive Trials + Impetus = A Perfect Match
 Impetus Digital can help at all stages of adaptive trial planning, execution, and reporting. For example, the Impetus InSite Platform® can be used to easily share knowledge among, and gather insights from, investigators or statisticians that have previously worked on adaptive trials. You can also collect insights from oncology KOLs regarding their views on evidence from adaptive vs. traditional trials for treatment decision-making. Using the platform’s asynchronous annotation tool, all collaborators can effectively review study protocols and abstracts or articles for publication. The same tool is also useful for co-creating guidelines, for example regarding terminology and best practices in adaptive trials. Moreover, national or international online learning programs surrounding adaptive trial designs are an impactful and cost-effective way to educate key stakeholders.
Impetus Digital can help at all stages of adaptive trial planning, execution, and reporting. For example, the Impetus InSite Platform® can be used to easily share knowledge among, and gather insights from, investigators or statisticians that have previously worked on adaptive trials. You can also collect insights from oncology KOLs regarding their views on evidence from adaptive vs. traditional trials for treatment decision-making. Using the platform’s asynchronous annotation tool, all collaborators can effectively review study protocols and abstracts or articles for publication. The same tool is also useful for co-creating guidelines, for example regarding terminology and best practices in adaptive trials. Moreover, national or international online learning programs surrounding adaptive trial designs are an impactful and cost-effective way to educate key stakeholders.
The above are just a handful of examples – the possibilities really are endless!
Stay Tuned…
In part 2 of this series, I will discuss several specific types of adaptive and innovative trials in more detail. Much like adaptive trials in general, each of these associates with their own important pros and cons. In their own different way, each will play an essential role in creating truly personalized medicine for patients with cancer.
References
Berry, D.A. (2011). Adaptive Clinical Trials: The Promise and the Caution. J. Clin. Oncol., 6, 606-609.
Berry, D.A. (2015). The Brave New World of clinical cancer research: Adaptive biomarker‐driven trials integrating clinical practice with clinical research. Mol. Oncol., 9, 951–959.
Pallman, P., Bedding, A.W., Choodari-Oskooei, B., et al. (2018). Adaptive designs in clinical trials: why use them, and how to run and report them. BMC Medicine, 16, 29.
US FDA. (2018). Guidance Document – Adaptive Design Clinical Trials for Drugs and Biologics. Retrieved from https://www.fda.gov/downloads/Drugs/Guidances/ucm201790.pdf


