With many patents about to expire in coming years, the field of biologics in oncology is about to change dramatically. Biologics are predicted to comprise over one-quarter of the pharmaceutical market by 2020. Currently, almost half of all biologics sales come from 11 major agents. All of these will have their patent expire in the next few years. This, along with worldwide efforts to provide value-based treatment and improve access to more affordable healthcare, presents an attractive opportunity for biosimilars.
Biosimilars—More Than Just Generic Drugs
Whereas generics are chemically identical products to the reference drug, with relatively simple chemical synthesis and negligible impact of any differences in the manufacturing process, biosimilars are a lot more complicated.
To understand biosimilars, we first have to understand the concept of biologics. Biologics are typically large, complex molecules that are created using living systems such as bacteria or mammalian cell lines. Due to the complex, multi-stage manufacturing process, which involves both maintenance of the cell line as well as isolation and purification of the product itself, there is unavoidable variability in the manufacturing of biologics. For this reason, and because many details of the manufacturing process remain trade secrets, creating generic, identical versions of biologics is often not possible.
Instead, attempts to recreate the reference product may result in non-clinically significant differences in molecular structure due to slight variations in the manufacturing process – that is, in biosimilars. The FDA defines biosimilars as biologic products that are highly similar to the reference product, notwithstanding minor differences in clinically inactive components, and without clinically meaningful differences in terms of safety, purity, and potency.
A Brief History of Biosimilars
The EMA first established a separate pathway and legal framework for approval of biosimilars in the mid-2000s. Subsequently, this has served as the basis for regulatory guidelines throughout the world. Subsequently, the first biosimilar was approved in the European Union in 2006. In the US, the FDA has historically had limited authority to review and approve biosimilars. This has led to delays in their availability. However, the 2010 Affordable Care Act enabled the FDA to develop an approval pathway for biosimilars through an abbreviated review process. As a result, in 2012, draft guidance documents were released, providing clear guidelines on the allowed variability.
Current Status
While the use of biosimilars is expanding rapidly in Europe, the US market is showing much slower growth. As of mid-2018, 11 biosimilars were approved by the FDA, including 4 in the oncology space. However, most are not actively marketed due to either being tied up in patent disputes or awaiting patent expiry. Consequently, the time-to-market for many biosimilars is likely to be extended.
Biologics in Oncology
There are currently several different types of biologics in oncology. These include:
- Immune checkpoint inhibitors
- Immune cell therapies, such as tumor-infiltrating lymphocytes and CAR-T cell therapy
- Therapeutic antibodies
- Therapeutic vaccines
- Immune-modulating agents such as cytokines
We have previously touched on immune-oncology drugs here, as well as the role of targeted therapies such as therapeutic antibodies in personalized medicine here. While we will not be going into detail about the remaining types today (but watch this space in the coming months!), there is no doubt that biologics represent both the present and the future of cancer therapy.
Biosimilars in Oncology
Irrespective of the type, one thing that most, if not all, biologics have in common is their high cost. The introduction of biosimilars is generally viewed as an economically beneficial move, which should theoretically lower the price of biologics treatment due to direct competition with the original biologic agent. In fact, a recent analysis estimated that the availability of biosimilars could reduce direct healthcare spending by $54 billion between 2017 and 2026. Consequently, biosimilars are also expected to promote increased access and use of biologics in oncology.
As mentioned, the FDA has approved 4 biosimilars for cancer treatment to date in the US. These include filgrastim-sndz (Zarxio), pegfilgrastim-jmdb (Fulphila), trastuzumab-dkst (Ogivri), and bevacizumab-awwb (Mvasi) as biosimilars to filgrastim (Neupogen), pegfilgrastim (Neulasta), trastuzumab (Herceptin), and bevacizumab (Avastin), respectively. Many more are in development or are awaiting approval.
Some major players (such as Sandoz, Amgen, and Pfizer to just name a few) have set up programs aimed specifically at developing biosimilars. However, there are significant differences in the regulatory and development processes between generics and biosimilars, which may provide important challenges for biosimilar approval and adoption. Accordingly, for biosimilar manufacturers to move forward and navigate the complex regulatory processes, it is imperative that oncologists and other key stakeholders understand how biosimilars are regulated and approved, as well as their cost-effectiveness compared to their reference product.
Barriers to Biosimilars in Oncology
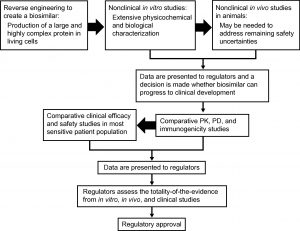
Obtaining Approval for Biosimilars
The approval process for a biosimilar comprises multiple stages, and ongoing communication between the manufacturer and the FDA or other regulatory agencies is needed throughout. As a first step, the comparability between the biosimilar and reference product needs to be established. To this end, both structural and functional analyses are needed. The extensiveness and robustness of these analyses and the data they generate will dictate the need for subsequent animal toxicology and human clinical studies. Additionally, pharmacological studies demonstrating bioequivalence (including in pharmacokinetics and pharmacodynamics) and studies evaluating the immunogenicity of the biosimilar must be performed, followed by a clinical trial in the most sensitive indication approved for the reference product to identify potential differences, using an equivalence or non-inferiority trial design.
It is worth noting that the primary endpoints in biosimilar clinical trials are usually chosen to detect clinically relevant differences compared to the reference product, and may thus differ from those used for the approval of the original product. As the preferred endpoint to prove efficacy in oncology trials (e.g. PFS or OS) may not be sensitive enough to demonstrate biosimilarity, the EMA recommends using a primary endpoint that measures activity, such as ORR or pCR.
Indication Extrapolation, Immunogenicity, and Post-approval Safety Monitoring
Once evidence of bioequivalence and safety has been established and a biosimilar is approved based on the “most sensitive” indication, it can be used for all indications of the original product. However, this indication extrapolation has raised some concerns, and to address any potential efficacy or safety issues in other patient populations, a pharmacovigilance program must be initiated during the post-approval period. Moreover, clinically meaningful immune responses to biologics and biosimilars may develop after long-term use, potentially affecting both their safety and efficacy. Having a solid pharmacovigilance program in place will thus also help identify rare but potentially serious safety risks such as immunogenicity that were not detected during the clinical trials.
Need for Global Harmonization
With the increasing biosimilar development and integration into healthcare markets, global harmonization of the standards for developing and approving biosimilars is needed. In turn, this will speed up the approval process and improve accessibility.
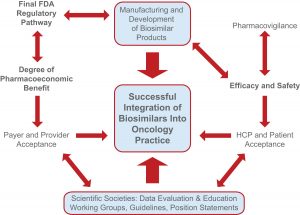
Clinician Acceptance of Biosimilars
Another important barrier to biosimilar use in oncology is clinician acceptance. Oncologists, together with other healthcare providers such as pharmacists and oncology nurses, are key to ensuring the uptake of biosimilars in clinical practice. While the lower price compared to the original product will be a key driver, some clinicians may cite the lack of clinical studies on the biosimilar as a reason for continuing using the reference product. In the absence of sufficient data, especially for indications and patient populations where the biosimilar data were extrapolated based on the original product, clinicians may hesitate to prescribe a biosimilar. In addition, there may also be concerns regarding the impact biosimilars will have on reimbursement.
Overcoming the Barriers to Biosimilars in Oncology
Industry Support and Development of Streamlined Study Approaches
The development of new guidance for the use of bioanalytical and pharmacokinetic/pharmacodynamic modeling data is one way to facilitate the development of biosimilars. Moreover, introduction of lower burdens of proof in clinical trials will also be helpful. To achieve this, industry support will be needed. Furthermore, new, more time- and cost-effective, and streamlined approaches for clinical trials and patient recruitment should be identified. For example, placing more emphasis on real-world evidence, forming joint ventures with other companies, and leveraging healthcare providers and patient advocacy groups may all help drive uptake.
Medical and Patient Education for Ensuring Acceptance of Biosimilars
To overcome the barrier of reluctance in using biosimilars, education will be key. This includes for oncologists, other healthcare providers, patients, and payers. In fact, surveys have shown that the overall knowledge about biosimilars in the medical community is somewhat limited and that more information is required for oncologists to feel comfortable prescribing them. Accordingly, accumulation of strong clinical data and CME featuring unbiased experts will be invaluable to educate oncologists on biosimilars. In turn, this will help facilitate informed decision-making, promote acceptance of biosimilars in clinical practice, and accelerate the associated health and economic benefits.
 Conversely, patients may be drawn to biosimilars due to their lower price, especially if they are paying out-of-pocket. The growing role of patients and patient groups in treatment decisions should therefore not be underestimated. Nevertheless, education is needed for patients too to make them comprehend the differences between the various treatments.
Conversely, patients may be drawn to biosimilars due to their lower price, especially if they are paying out-of-pocket. The growing role of patients and patient groups in treatment decisions should therefore not be underestimated. Nevertheless, education is needed for patients too to make them comprehend the differences between the various treatments.
Importance of Ongoing Relationships with Key Opinion Leaders in Oncology
Biosimilars offer significant opportunities for expanding access to populations that need these therapies but cannot afford or access the original. Hence, clinician outreach and pricing policies go hand-in-hand. Furthermore, a commercial strategy closely resembling the launch of an original biologic is critical. Direct access to stakeholders who can impact decisions and help gain wide acceptance of the product is imperative. Accordingly, identifying and connecting with the right stakeholders is needed to compete with the reference product or within the biosimilar space.
How Impetus Can Help
The Impetus InSite Platform serves multiple purposes and can be used to engage with stakeholders at all phases of biosimilar development, approval, and post-approval marketing and surveillance. In addition to collecting important insights from oncologists and other key opinion leaders, the platform’s asynchronous annotation and selection tools can also be used to co-create educational materials for healthcare professionals and patients with advisors or working group members spread throughout the country or world.
Moreover, the Impetus InSite Platform can also be used to deliver medical education on biosimilars to healthcare professionals, delivered in the form of asynchronous but interactive discussions on new clinical or real-world data, online debates, case studies, and/or real-time webinars. This is a cost-effective means of reaching large numbers of participants in different geographic regions. As mentioned, most oncologists will base their treatment decisions on safety and efficacy data from clinical trials. Therefore, instilling knowledge about a product or about biosimilars in general is essential.
Concluding Remarks
Despite uncertainties about their long-term safety, indications, and reimbursement, biosimilars undoubtedly have the potential to increase access and lower costs for biologic treatments in oncology. Regulatory requirements and global harmonization/standardization strategies are evolving, which will eventually facilitate their clinical development. However, ongoing education to oncologists and other stakeholders will be key to ensure uptake, along with carefully designed marketing strategies.
References
Chen, Y., Dikan, J., Heller, J., Santos da Silva, J. (2018). Five things to know about biosimilars right now. McKinsey. Retrieved from https://www.mckinsey.com/industries/pharmaceuticals-and-medical-products/our-insights/five-things-to-know-about-biosimilars-right-now
Cohen, J. (2018). What’s Holding Back Market Uptake Of Biosimilars? Forbes. Retrieved from https://www.forbes.com/sites/joshuacohen/2018/06/20/whats-holding-back-market-uptake-of-biosimilars/#5d8997ef691a
Nabhan, C., Parsad, S., Mato, A.R., Feinberg, B.A. (2018). Biosimilars in Oncology in the United States: A Review. JAMA Oncol, 4, 241-247.
National Cancer Institute. (2018). Biological Therapies for Cancer. Retrieved from https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/bio-therapies-fact-sheet
Rak Tkaczuk, K.H., Allen Jacobs, I. (2014). Biosimilars in Oncology: From Development to Clinical Practice. Sem Oncol, 41, S3-S12.
Rugo, H.S., Linton, K.M., Cervi, P., Rosenberg, J.A., Jacobs, I. (2016). A clinician’s guide to biosimilars in oncology. Cancer Treat Rev, 46, 73-79.
Stenger, M. (2018). Opportunities, Issues, and Challenges for Biosimilars in Oncology. The ASCO Post. Retrieved from http://www.ascopost.com/issues/july-25-2018/opportunities-issues-and-challenges-for-biosimilars-in-oncology/
Truven Health Analytics. (2017). The Importance of Early Key Opinion Leader Outreach in the Biologics and Biosimilars Marketplace. Retrieved from https://truvenhealth.com/Portals/0/assets/2017_Truven_Health_Early_KOL_Outreach_Biologics_Biosimilars.pdf
Zelenetz, A.D. (2016). Biosimilars in Oncology. touchONCOLOGY. Retrieved from: https://www.touchoncology.com/articles/biosimilars-oncology